The science of butyrate and weight loss
And how to read the medical literature
The following is not medical advice. It is scientific information. Talk to your doctor before you make any changes to your lifestyle, medications, or supplements that might affect your health.
Announcement
Become a FOUNDING subscriber and get access to a growing list of discounts and deals that I have made available via partnerships with companies whose products I use.
I will make my post that will give founding members this information today.
Without further ado…
A new study
What if you could:
3 points BMI reduction (~15 pounds in someone who is 5'0")
5 cm waist circumference reduction
Halve fasting insulin
Halving inflammatory markers
all in just one pill?
Butyrate has many well-documented biological effects in a laboratory context, and it is just starting to be tested for its potential benefits in humans.
For the first time, after over a decade documenting a similar effect in laboratory animals, a paper published in JAMA Network Open showed a strong weight loss benefit of butyrate supplementation for overweight and obese children. Link.
The basic science of butyrate
Before I discuss the study, I want to first introduce the basic science of butyrate: what it is, how the body produces it, what effects it has.
If you just want to know what the paper in JAMA said, skip to the section that says OBESITY, below.
Otherwise, strap in to learn about one of the coolest metabolites in the mammalian organism.
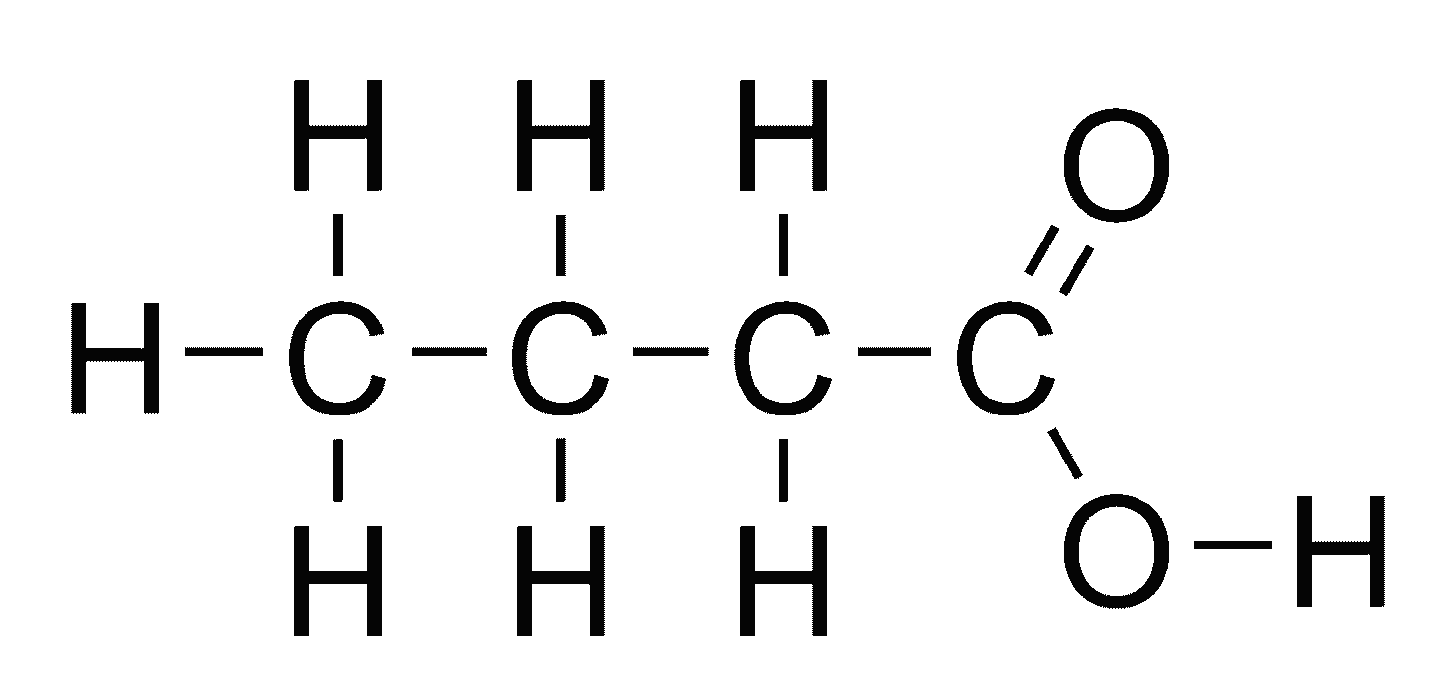
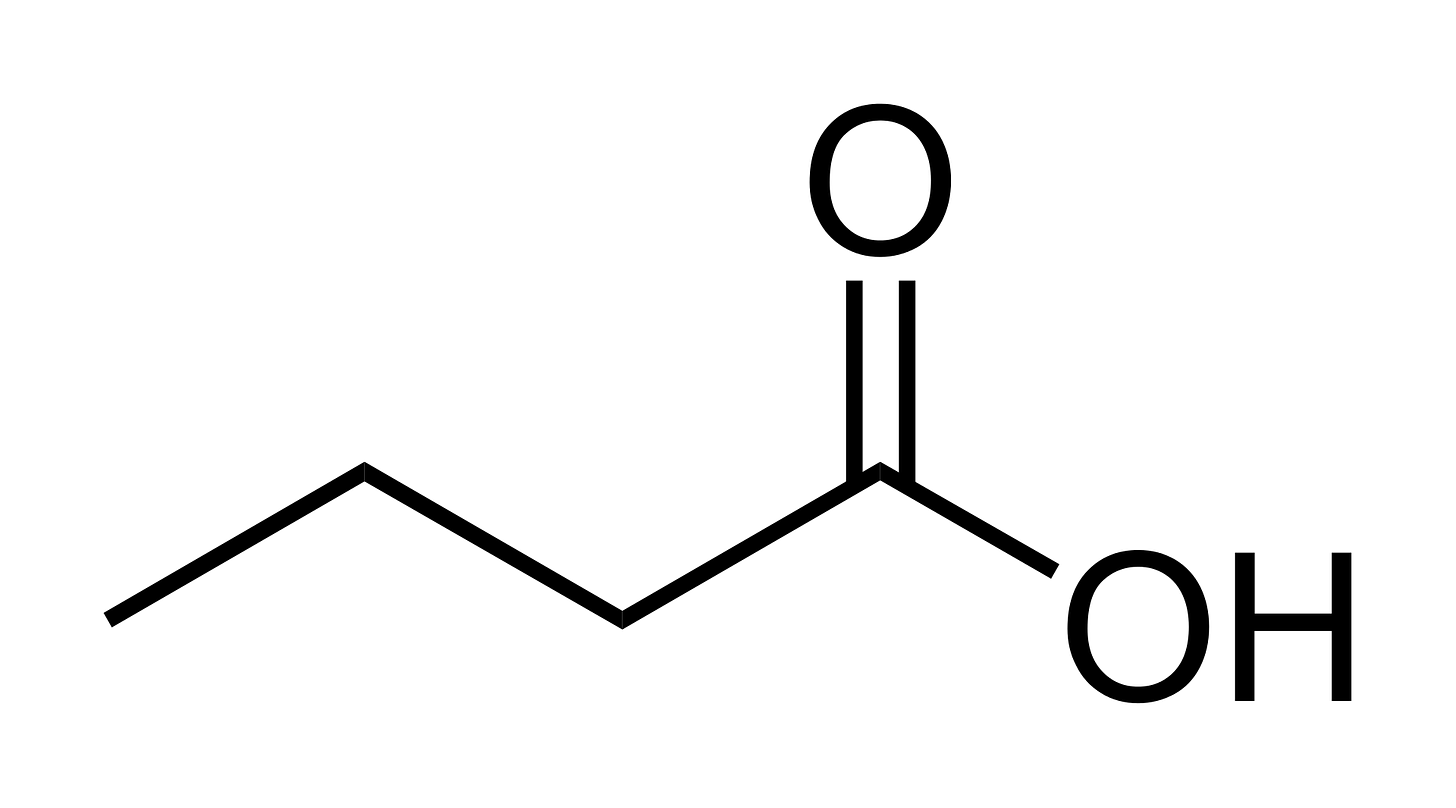
Butyrate is known as a short-chain fatty acid, or SCFA, with a 4-carbon chain.
Here are two representations of butyrate:
Butyrate is a fatty acid, which is what the molecules in fat are: they are all fatty acids. But it is a SHORT-CHAIN fatty acid because, the length of the chain is short, as you can see here in these two representations.
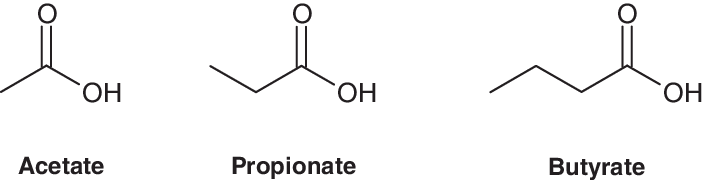
Other SCFAs include acetate and propionate, which are SCFAs with 2- and 3-carbon chains, respectively. So that you can see what I mean by this, here is a representation of acetate, propionate, and butyrate, side-by-side:
Compare these, to say, stearic acid, a long-chain fatty acid (or LCFA), found in beef (and chocolate):
That's why these are called short-chain fatty acids, or SCFAs.
Now the SCFAs acetate, propionate, and butyrate are produced by the human colon in a roughly 60:25:15 ratio, though this depends somewhat the diet consumed by the individual in question, as well as other genetic, lifestyle, and environmental factors.
Now back to butyrate specifically. The name butyrate is derived from the ancient Greek βούτῡρον (bouturon), which means "butter".
That's because butter is particularly rich in butyrate (3-4%), and indeed, butyrate smells very strongly like butter. Indeed, butyrate is responsible for the distinctive smell of butter, as discovered in 1815.
That buttery smell and flavor in butter? Caused by butyrate. Which is why some processed foods have butyrate added directly, in order to simulate the taste of butter directly. Who knew!
Now in the adult mammal, butyrate is produced in the gut from the fermentation of fiber by bacteria. And it is produced by the fermentation of fiber by bacteria in the gut.
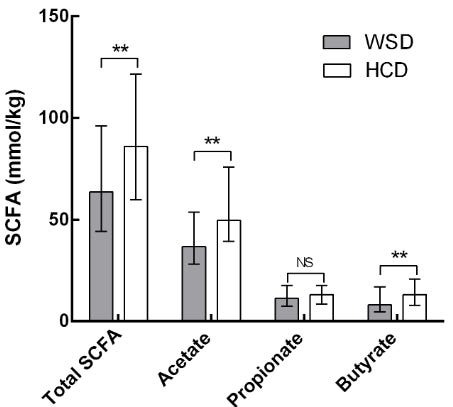
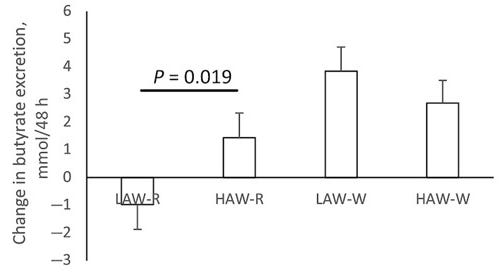
Therefore, as expected, a high-fiber diet can substantially increase butyrate production. This has been shown in both mice and in humans. Link.
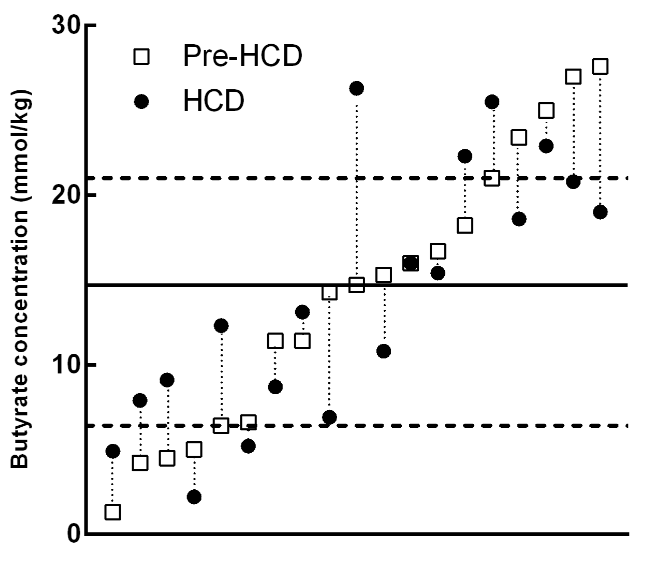
However, for whatever reason, there does seem to be some variability in the amount that butyrate production increases in response to changes in dietary fiber intake. Here we see that the high-fiber diet (HCD) does not produce more butyrate in many people. Link.
As we can see, those with the highest baseline butyrate concentrations tend to see reductions in butyrate on consuming the high-fiber diet, while those with the lowest tend to see increases.
Still, there was an overall increase in fecal butyrate in the HCD group (a doubling):
Another study confirms this finding, showing higher butyrate concentrations for the higher-fiber diets (second, third, and fourth here).
So what's butyrate good for? Well, a few things. We're not going to cover everything in this post, because there's a lot.
But one thing we know is that butyrate is the preferred energy substrate of colonocytes--the cells that line the colon.
In fact since 1982 we have known that colonocytes preferentially use butyrate--above all other sources of energy found in the colon, including glucose. Link.
And we know that in many contexts, colonocytes don't even use energy sources apart from butyrate, at all. Link.
And that in germ-free mice without butyrate, the colonocytes are abnormal, but they are normalized by adding butyrate. Link.
And indeed in several animal models of colon cancer, butyrate slows and/or prevents progression. Link.
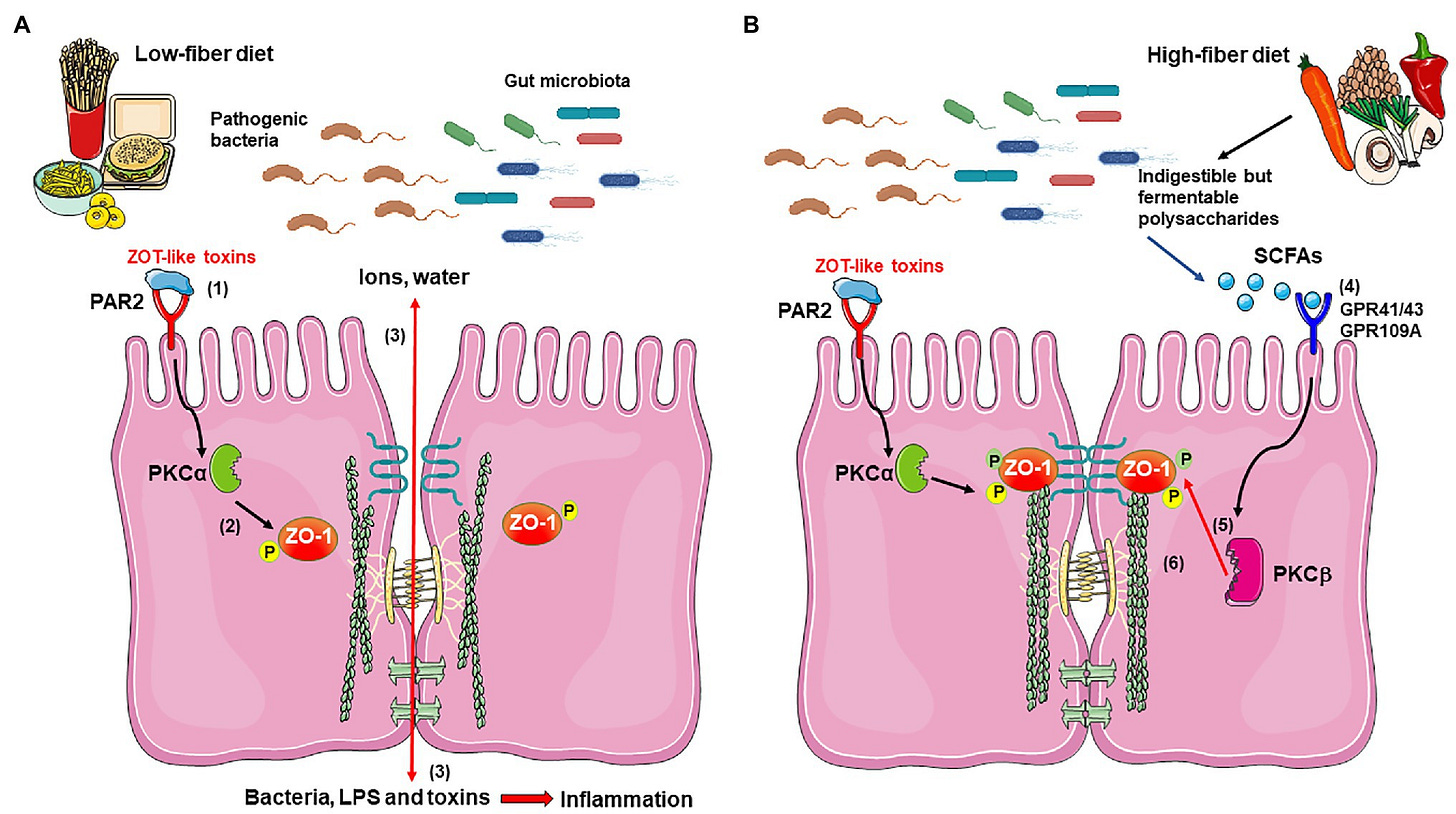
Butyrate in the colon also enhances the mucosal barrier between the intestine and the blood by increasing the assembly of something called tight junction proteins--which help to "seal" the intestinal barrier. Link 1. Link 2.
Butyrate therefore likely serves a very important function in gut immunity, as well as whole-body inflammation--as we will see later--by tamping down the exposure of the intestinal contents to the systemic circulation by reducing intestinal permeability.
Butyrate may have these beneficial effects on the gut by signaling to the gut that everything is "working properly"--that the right microbes are present in the gut, making the right metabolites, and it's OK therefore for the immune system to "stand down".
Because if butyrate isn't being produced, that's a real problem and indicates the possibility of severe disruption of normal microbial activity--and possible infection.
And this may be why diets low in fiber may contribute to chronic systemic inflammation. Some evidence supporting this hypothesis comes from fiber supplementation studies, which very commonly show reductions in inflammatory biomarkers. Link.
In any case, it is thought by experimentalists that fiber--and the metabolite butyrate that is produced from it--is probably very important to the health of the colon, for the reasons explained above (and so many others that I haven't mentioned here).
And this links to the butter story. Butter comes from milk, and milk contains butyrate.
Why does milk contain butyrate?
Possibly because, before children can eat whole foods that can produce butyrate in the colon, they need a ready source of butyrate, so it comes from milk!
(An aside--this is just a theory based on laboratory evidence. To know that things really work this way in humans, we multiple new clinical trials--that would monitor various health outcomes. We only have a small part of those so far.)
OK, so that's the colon health story.
But what about obesity?
Obesity
Well, for reasons that are still unclear, when butyrate interacts with the gut upon ingestion, there are strong anti-obesogenic effects too: butyrate suppresses appetite and causes weight loss.
Here are three figures from three separate mouse studies showing the impact of acetate, propionate, butyrate, and fructo-oligosaccharides (FOS; a fiber that ferments into SCFAs) on weight in young mice. Link 1. Link 2. Link 3.
There is a large impact.
These data are the basis of this trial in humans in JAMA Network Open. Link.
Before I discuss the trial, I want to briefly explain what a randomized controlled trial is, and why it is the gold standard in medicine, and why it is so important to know whether something is a randomized controlled trial or some other design.
While the studies I have linked in the first part of this post in mice and in cells are important for exploring and elucidating new biological mechanisms, and generating ideas that can drive new potential treatments, they are not a real test of that treatment in humans.
This is for a lot of reasons. One is that in mouse and cell studies are often pretty untrustworthy due to the lack of oversight and standardization and the perverse incentives that are involved in conducting these studies (a post in itself).
Another reason, of course, is the species or model difference: what works in mice or even in human cells may not work in an actual human.
In fact, in an actual human, there may be unexpected consequences that were not detected in earlier experiments in cells or mice.
That's why we test using a randomized controlled trial. A randomized controlled trial takes a group of people and randomly assigns them to two or more groups (usually two).
One of these groups is a placebo group, the other is an intervention group.
It's very important to understand why randomizing is so important and has been such a breakthrough for scientific medicine:
Randomly assigning people to the treatment or placebo ensures that the odds that the two groups that are being compared are any different at the beginning of the study in a way that might affect the outcome is as close to zero as possible.
Onto the design of the study.
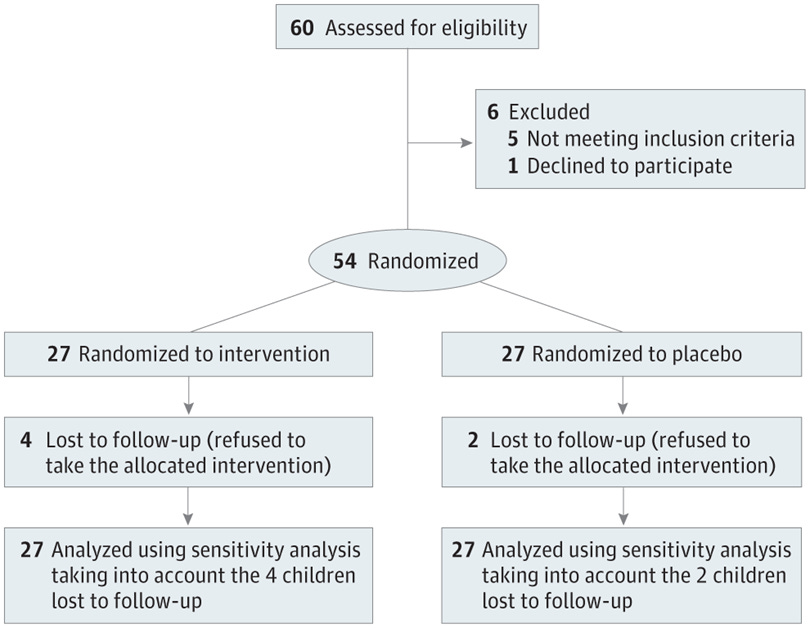
For the study that we are discussing from JAMA Open Network:
There were 54 obese children, ALL provided standard of care.
THEN:
27 were assigned to take a daily placebo: a capsule of cornstarch.
27 were assigned to take the intervention: a capsule of butyrate.
They did this for 6 months.
What were the results?
Check out the highlighted rows in this table:
Summary:
3 points BMI weight loss (~15 pounds in someone who is 5'0")
5 cm waist circumference reduction
Nearly halving fasting insulin
Nearly halving HOMA-IR
Nearly halving ghrelin (!)
Nearly halving IL-6
with relatively negligible changes in the placebo group
With very impressive results like these, I often immediately go to the trial registration at clinicaltrials.gov to do a first-pass quality check, to make sure I am not being fooled.
Here is the registration for this study: link.
The most important thing I look up is the primary and secondary outcomes and make sure that they match the study primary and secondary outcomes that are reported in the paper.
This ensures the investigators run the trial as planned and don't switch outcomes in the middle of the trial to make something that, by chance, ends up looking like a signal the outcome--basically, to "cook" the data to make it say something it really doesn't.
For this study, they do match:
So, altogether, this is wild! It suggests that the effect of butyrate on weight is very similar to that seen in mice.
And the reduction in IL-6, a classic obesity-related inflammatory marker, suggests that there might be anti-inflammatory effects (possibly related to gut permeability), too.
This part however should be interpreted cautiously, since weight loss, by itself, independently reduces inflammatory markers. So the reduction in inflammatory markers may be due simply to the weight loss.
That said, the weight loss is huge. And, interestingly, when I go back to the original mouse studies and look at the control diets, they are standard chow diets, that is, diets that are unrefined and high in natural fiber.
This implies that supplemental butyrate may exert these effects even on the backdrop of a high-fiber diet.
What about safety and adverse events? None reported by the investigators apart from in the first month.
What we need now is larger studies looking at harder clinical outcomes. Not just weight loss but rates of cardiovascular disease, cancer, death. Such studies will help to confirm the salutary impact of butyrate supplementation on health.
But these results are promising.
We cannot make broad-based recommendations, but those of us who are curious can try. And as someone with 10-15 pounds I would like to lose, I'm going to do it.
Become a paid subscriber to access an extended discussion on this post that includes the following:
A further discussion of the randomized controlled trial and its potential applications for the rest of us;
A list of foods that contain fiber that cause butyrate to increase;
A list of fiber-containing supplements that increase butyrate naturally (with links and exclusive discounts);
A list of butyrate-containing supplements (with links);
Dosages that are consistent with benefits in the literature.