Replimune, FDA, and a skewering of the Wall Street Journal, by yours truly
If you’ve been following the drama, reported here on this Substack and on X, Wall Street Journal seems to have a real problem with FDA, having recently piggybacked on Laura Loomer’s cancelation attempt, writing two separate articles denouncing Trump’s FDA and its officials Marty Makary and Vinay Prasad.
Prasad was canceled—temporarily. Then he was reinstated. Did I help? I’m told by some well-connected individuals that I did. I certainly upset Laura Loomer, who threatened to sue me and Ned Ryun.
However, everything that I said was also said by White House officials to The Free Press. So Loomer is likely to have a hard time of it. Pharma’s fingerprints are all over this. Loomer was way too obvious. I still haven’t received the notification of the lawsuit. I’ll be waiting. (Not really.)
Read the last post if you are interested in that background.
Now, Wall Street Journal has launched another dumb attack—this time at Trump FDA appointee Marty Makary.
This article is filled with wild representations.
This post will explain.
I also explain the role of science in medicine and why WSJ Editorial Board is not smart.
WSJ is very dumb lately, and I believe that this is for an obvious reason: the people who are writing for it are dumb.
This thread will explain the dumb.
I implore you, dear reader:
As I explain, revel in the dumb. Wonder at it. Laugh at it.
Be not perturbed by the dumb.
The Editorial Board opens with an accusation: Makary is "torpedoeing" "life-saving drugs", which "raises big questions".
This is completely wrong.
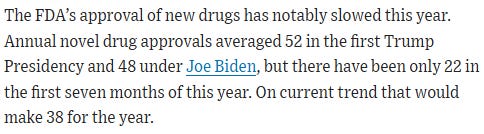
Next, the Board accuses Makary of slowing the approval of new drugs.
Once again, wrong.
There were 41 approvals in 2022, 55 in 2023. It fluctuates.
Plus, approvals accelerate in the second half of the year.
FDA is set to beat 2022, especially as the agency settles.
In the next paragraph, the Board gets downright nasty, accusing the "regulators [of] nixing drugs under the false flag of raising scientific standards."
WSJ Board cites Replimune, claiming that a third of cancer patients benefited from the drug.
And now we get started.
We need to place this discussion in context.
In 2011, a JAMA IM paper analyzed all clinical trials published in 2009 in New England Journal of Medicine that tested standard medical practices.
46% of practices were found to be worthless.
Another published in 2011 examined all trials in the NEJM published from 2001 to 2010.
This time, 40% (146 of 363) of the trials testing standard of medical care showed these standard practices to be worthless.
In 2019, another JAMA IM paper showed that of drugs approved along the FDA accelerated approval pathway for 93 cancers over the previous two decades, only 1-in-5 were shown to have actual benefit to patient survival.
80% hadn't.
In 2021, a BMJ paper analyzed drugs for 18 cancers that had undergone FDA accelerated approval but had failed to show benefit in follow-up studies.
A third of these drugs continued to be recommended in guidelines, sometimes even after FDA approval had been revoked.
Cancer drugs? Going through FDA accelerated approval?
Does that sound like the Replimune?
It sure does.
Replimune submitted a single-arm trial of its cancer drug to FDA for accelerated approval.
Now that's a big problem. It's a trial without a control group.
"But 1/3 of patients got better, Dr. Bass!" you might say. "They're cancer patients. A third of patients with advanced melanoma don't just get better on their own. Come on now."
And you'd be right. Except just one thing: they were also given another drug along with Replimune.
"WHAT?! The Editorial Board left out that detail?! They misled us like that?"
It gets worse.
The patients enrolled in the trial were exceptionally healthy, increasing their odds of responding.
And remember how the patients "hadn't responded to prior immunotherapy"?
Oh boy.
It turns out to be enrolled in Replimune's trial and be considered "non-responsive", you had to undergo eight weeks of immunotherapy.
And do ya know how long it takes for immunotherapy to start working?
Then the patients were slapped right back on immunotherapy again, along with Replimune's drug.
Right when immunotherapy starts working.
"No, that's impossible!" you say, "People cannot be defending this!"
Oh but as you can see, they are.
It gets worse.
When the WSJ Editorial Board harped on "a third of patients responded"?
The drug given along with Replimune achieves similar results by itself:
CheckMate 066 (nivolumab alone): 40% ORR
CheckMate 067 (nivolumab alone): 44% ORR
And when you give nivolumab along with other cancer drugs (not Replimune), the results are even more striking:
CheckMate 067 (nivolumab + ipilimumab): ORR 58%
That's nearly DOUBLE the response rate as the Replimune trial.
Oh it gets worse.
Because as it turns out...
When you don't "design" the trial like Replimune did with just 8 weeks of immunotherapy before enrollment, and you design it at least 24 weeks of immunotherapy, where one might really make the argument that the cancer really was treatment-resistant...
Other trials have gotten nearly identical results as Replimune's.
That is, Replimune was used alongside nivolumab in a cooked study design.
But even with a huge disadvantage, you get the same results:
Avance (pembrolizumab + ipilimumab): 29% ORR
WSJ's Editorial Board exclaims: "tumors shrank in nearly all patients, and responses proved durable over three years."
Oncologists "hailed the results".
Then they insinuate that FDA is being unreasonable.
But now you know the real story: WSJ has apparently become a rag.
Here's the next paragraph. You now know why this is ridiculous.
"Its quibble is that the trial lacked a control group."
It's really hard to take this level of ignorance seriously.
Moreover, it's unclear why WSJ is attacking Prasad again.
It's widely known that Prasad wasn't even involved in the decision and had been for more than a week before the WSJ piece.
Here is StatNews saying exactly that:
In fact, Prasad, who was ousted from his FDA leadership post last week after a series of conservative figures criticized decisions of his that blocked the approval of new medicines, played no substantive role in the Replimune decision.
The company’s drug was expected to secure approval as a new treatment for patients with advanced skin cancer no longer responsive to currently approved medicines, including immunotherapy. But on July 22, Replimune said it was surprised to learn the FDA had turned it away, leaving its future in limbo.
“This was Rick Pazdur’s doing,” one of the FDA officials told STAT. “I didn’t agree with all the decisions made here by Vinay [Prasad], but he had little to do with this one.”
A second FDA official said CBER mishandled the RP1 review from the beginning, forcing Pazdur and his team to get involved late in the process. The situation was exacerbated by leadership upheaval within CBER, the official said, adding that Prasad tried to moderate the debate between the two sides as best he could.
Yet WSJ printed it anyway.
Why? It's probably because they are trying to build some kind of Prasad narrative, this good-vs-evil thing to distract from bad science. It's the only way I can explain it.
But here's the thing that really gets me:
“Never mind if patients die in the interim.”
If you don't do as I say, even when I'm saying nonsense, then you're responsible for deaths.
How did WSJ become like this? This sounds like the kinds of arguments made by the trans community for providing “gender affirming care” to children.
“If you don’t do this, if you question the evidence, people will die—nevermind that we haven’t shown that this is even true. We’re just going to say it.” It is completely crazy.
Still, it gets worse...
Next WSJ Editorial Board says something that betrays an incredible level of ignorance.
It is as if whoever wrote this piece merely mashed their face on the keyboard and used autocorrect to produce meaningful sounding sentences that are unhinged from any semblance of reality.
First of all, as we have seen, rechallenge with drugs actually does produce results with advanced melanoma.
Second, Replimune themselves have conducted or are conducting several such trials themselves, with CERPASS, IGNYTE-3, and REVEAL (links here, here, and here).
RCTs that Replimune cannot conduct because they are unethical... but which have been planned for years.
It's like WSJ cannot even do basic Google searches.
You need these trials, on tiny groups of people to make sure the drugs help and don't hurt people—before you deploy them to thousands of people.
You know, the point of clinical trials—to see if the drug works, because most cancer drugs don't?
Next paragraph we'll breeze through quickly, as it's mostly just opinion.
But we can see from the above research that I have linked that the endorsement by this Vishal Patel person just isn't true.
Likely a scientist who is not good at his job.
I skipped a paragraph (quote from another unhinged doctor/bad scientist).
But here's a real whopper. Apparently the Melanoma World Society president is even jumping in with the hyperbole.
I looked at the European Medicines Agency (the European FDA equivalent, which governs drug regulation in Germany) and I could not find anything indicating that the requirement for an RCT should be waived for Replimune. I found this.
The Replimune trial, as we have see is underwhelming and doesn't meet these standards.
I wonder what these people are thinking. I have no idea why people just randomly accuse other people of being unethical with no evidence just because they are emotional. It's really horrible.
WSJ then writes three more paragraphs about Prasad. They're so mad.
This is, as we have seen and as every other outlet indicates, Prasad had nothing to do with it.
Here:
medpagetoday.com/special-report…statnews.com/2025/08/04/rep…
But never let the truth get in the way of a good villain.
We should really ask a few meta-questions after all of this.
First of all, is this really the right way to talk about science: to take an emotional angle without concern for the facts? It is if you are a rag, but is this good for society, good for patients, good for science? We saw this approach during COVID. It's horrible for everyone.
Second, there's a deeper question here. Is accelerated approvals for various drugs really the way to spur innovation? What these pharma companies are mostly doing is applying technological advances that have already been made elsewhere.
If we care about innovation, the funding of fundamental scientific investigation needs to be reformed. Why doesn't WSJ write more about this, rather than this hysterical fake science journalism?
Third, how about biotech companies complaining less about "changing standards" at FDA and instead produce more than just bare minimum science that checks boxes but actually provides no scientific answers about whether their drugs work?
These companies are designing studies without any real concern whether their drugs actually work--without any real concern with whether they help people--and more to just push paper to satisfy the crappiest FDA standards that are now being tightened.
It's ironic. A company that is running badly designed studies--that it knows are bad--essentially elicited the sympathy of WSJ editorial board to write a half-baked and uninformed article about its drug "saving lives".
Meanwhile, the company itself doesn't even appear to be concerned that its drug is saving lives, and rather seems more concerned about doing the bare minimum to make money. Then it blames the regulators for not going along with the scheme.
It's so bad. It's really the height of hypocrisy if you think about it. People who actually want to save lives will do good science. People who want to make money will just check the boxes.
The company's study design really tells us all we need to know. They were just checking the boxes. They didn't care. And WSJ piled on with hysteria to distract the public from this basic fact. It's really despicable.
Bonus points. When Vinay Prasad was Loomered, one of the top Replimune executives reposted Loomer's post.
We know what this is really about. This isn't about patients. They're furious that it's no longer moneymaking as usual.
They lost.
The American people are winning.














Malory and Prasad are heroes for standing against Pharma’s mighty Wurlitzer machine.
"The people who are writing for WSJ are dumb"
Trusting medical doctors is more than dumb - it's life-threatening.