Health Science Sunday #1. The Bible of Diet and Blood Pressure, Part 1
A series on everything you wanted to know about diet and blood pressure
The more I wrote and researched this topic, the more fascinating I found it, and I wanted to share what I found. What was originally intended as a single article will therefore be separated into two or three parts.
I have struggled with high blood pressure for years. It runs through my mother’s side of the family. When I was 160 pounds, I consistently had pre-hypertension. And at 225 pounds, I still have pre-hypertension—and sometimes even hypertension.
Over a decade, it simply hasn’t improved, despite being extremely fit.
Years of trying blood pressure medications had little impact. I wasn’t willing to start multiple drugs or higher doses.
The more papers I read, the worse the scientific data looked: elevated blood pressure was a real danger to my long-term health, especially my brain and heart.
I’m a scientist, and I want to preserve my brain function for as long as possible. And I want to live a long life. My chronically elevated blood pressure was not helping.
So I tried something new.
Within just a few days of following a new approach, my blood pressure was normal for the first time in years.
The approach was entirely nutrition-based. No pills. Just food.
And not just food—but two smoothies a day. That’s it. No other changes.
Everyone knows that a good diet promotes health.
What few people know is how large and fast the impact can be.
I learned about this approach from the work that Stan Efferding does with athletes.
This series is a very deep dive into the basic and clinical sciences of blood pressure, and the basic and clinical science on the role played by diet.
In short, I situate Efferding’s practical insights deeply in the scientific literature—and extend upon them.
Part 1 contains the following sections:
Why optimizing blood pressure is so important for long-term health
Some important variables for optimizing blood pressure
What is the optimal blood pressure? National and international guidelines disagree.
Is it possible for blood pressure to be too low?
The evolutionary basis for optimal blood pressure in humans
Addressing a common but mistaken view of human health and evolution
What’s next
Why optimizing blood pressure is so important for long-term health
Heart disease is the leading cause of death in the United States.
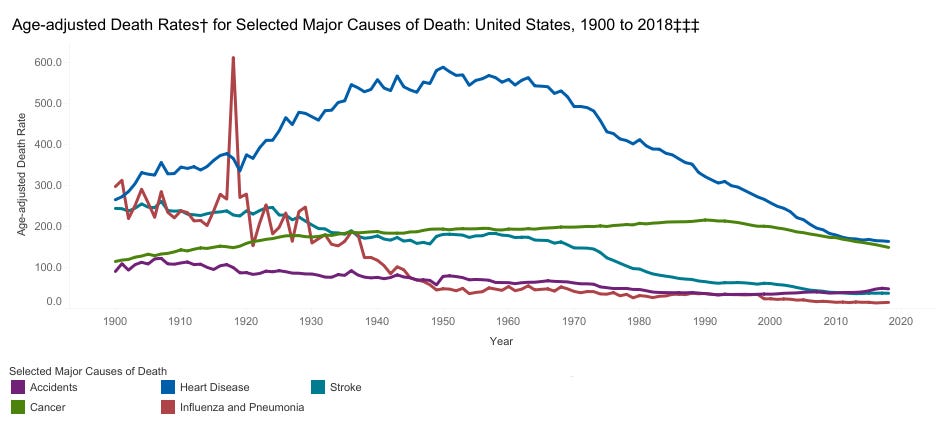
Now, there is a good reason why heart disease has plummeted so much over the past 70 years: Americans smoke much less. Smoking is about on par with diabetes for increasing risk of heart disease, each doubling it.
But after smoking and diabetes, blood pressure becomes one of the most important modifiable risk factors for cardiovascular disease. It is more important than elevated blood LDL cholesterol and even more important than abdominal obesity.
Indeed, as data aggregated from five large studies showed that, in men, simply having mild hypertension (SBP 140-149/90-99 mmHg) at the age of 45 doubles the risk of experiencing a cardiovascular event (fatal and nonfatal coronary heart disease, all forms of stroke, congestive heart failure, and other CVD deaths) by the age of 75, from 12% (with all risk factors optimal) to 25% (just with elevated blood pressure). Having severe hypertension (>160/100 mmHg) triples it.
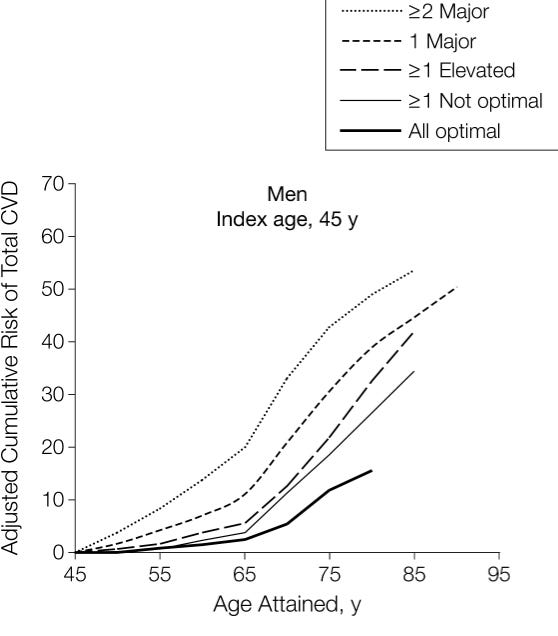
Experts estimate that nearly one-third of cardiovascular disease could be prevented just by getting everyone under 130 mmHg systolic blood pressure.
As we know from INTER-HEART, a study with data from 52 countries, hypertension interacts with other risk factors to increase their detrimental impact synergistically.
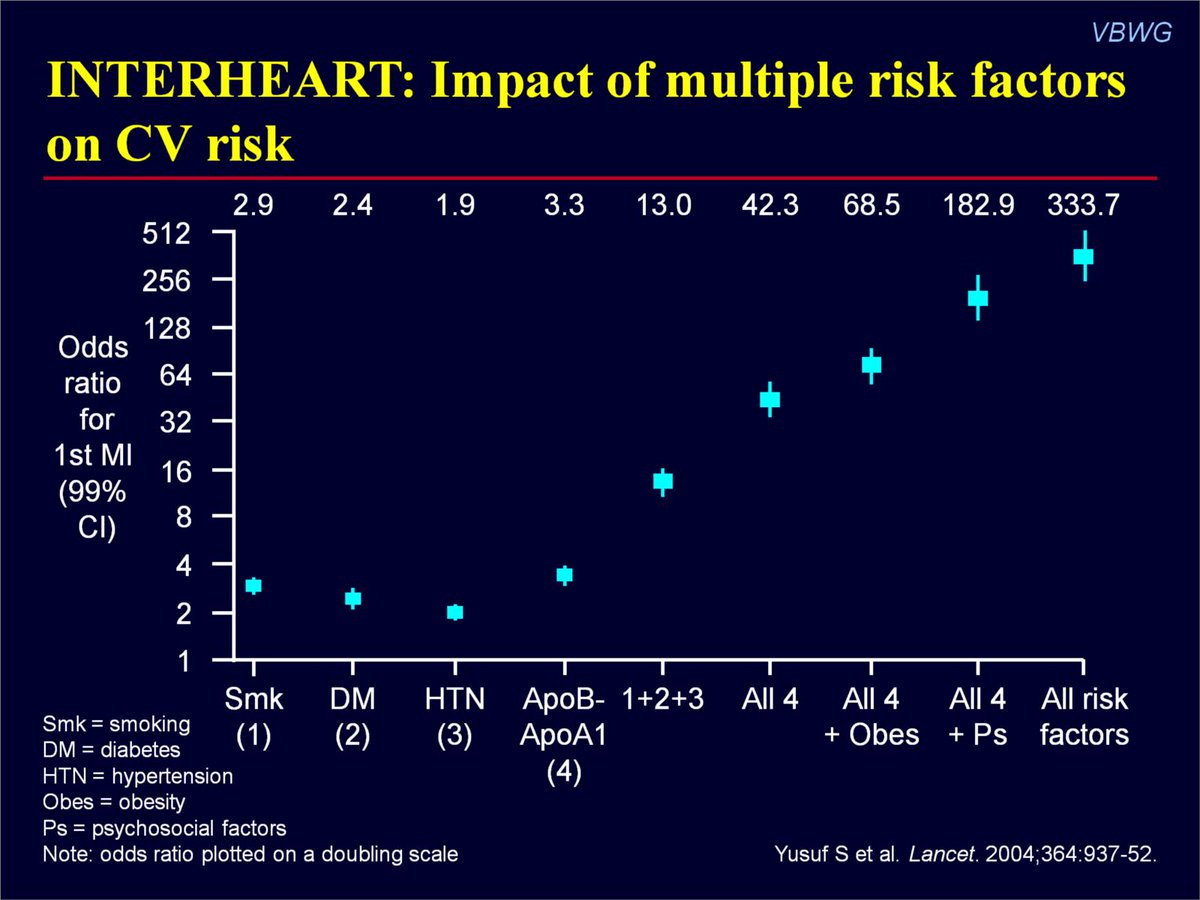
In other words, high blood pressure is not only harmful by itself—but becomes exponentially more harmful when combined with other risk factors.
Heart disease—which includes heart failure, atrial fibrillation, heart valve diseases, aortic syndromes, coronary heart disease and stroke—isn’t the only thing that elevated blood pressure can cause. High blood pressure in midlife also increases the risk of dementia, as well as the rate of cognitive decline. It also contributes to erectile dysfunction and female sexual function.
Keeping blood pressure optimal is best for heart, brain, and sexual function.
Some important variables for optimizing blood pressure
A number of lifestyle factors can have a significant impact on blood pressure.
Some of these include:
Body weight;
Sleep;
Caffeine or other drug intake;
Genetics.
(I do not here address nutritional factors, which will comprise several sections later.)
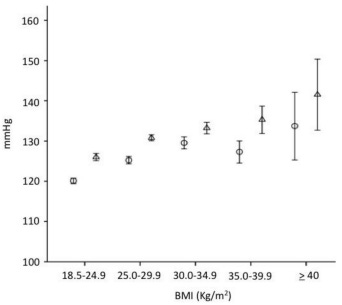
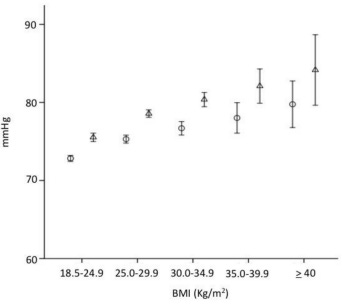
Let’s start with body weight. The relationship between body-mass index (BMI) and systolic and diastolic blood pressures has long been well-characterized:
That’s right. The average difference between a normal weight (18.5-24.9) and severely obese (≥40) is on the order of 15 mmHg systolic and 7 mmHg diastolic.
Yet, as we can see in the below figure, there is clearly much more to optimal blood pressure than just BMI:
The impact of sleep restriction is smaller but still substantial. In one randomized controlled trial, 1.5 fewer hours of sleep per night for 6 weeks led to an increase in 3.5 mmHg systolic and diastolic blood pressure.
The effect of caffeine is smaller: an increase of about 2.7 mmHg systolic and diastolic pressure after a week of adaptation.
The effect of amphetamine is much larger: on the order of 10 mmHg systolic blood pressure.
On average, the impact of oral contraceptive pills is significant but less than 1 mmHg systolic and diastolic blood pressure.
But the role of genetics is huge: the top 10% of humans with the highest genetic risk of high blood pressure had about 17 mmHg higher systolic and about 10 mmHg higher diastolic blood pressure than the bottom 10% of humans. That’s the difference between a blood pressure of 120/80 and 137/90 by genetics alone.
In other words, the genetic component of blood pressure is for some people larger even than the average blood pressure difference between having a normal weight and being severely obese!
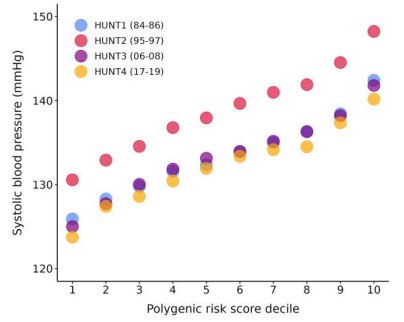
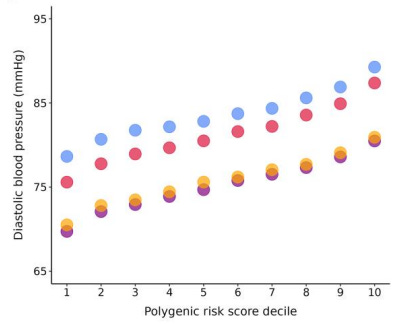
In other words, while one can certainly optimize blood pressure by simply optimizing the above lifestyle factors, genetics play a massive role.
What then?
This series is oriented toward answering that question.
Suffice to say, two approaches can be taken: optimizing lifestyle and using drugs.
It is my position that there is no fundamental difference between these two—and that lifestyle changes can have both similar benefits and risks as the use of drugs. Lifestyle changes are not always benign or without risk, which I believe I can and will conclusively in subsequent parts of this series.
What is the optimal blood pressure? National and international guidelines disagree.
But what blood pressure, exactly, is “optimal”?
This question is more difficult to answer than one might suppose.
We could, after all, simply look at the available guidelines to determine our optimal blood pressure target.
But this is a problem, because the available guidelines disagree.
In 2017, the American Heart Association, the American College of Cardiology, and eight other minor American professional organizations revised their guidelines: the blood pressure cutoff for the diagnosis of hypertension became 130/80: any blood pressure reading higher than 130/80 is diagnosed as hypertension and treated with lifestyle and/or medication.
Before this change, the cutoff was 140/90.
Yet this change is out of step with the guidelines from other countries, and even with the guidelines of some organizations in the United States.
The following organizations maintain a blood pressure target of 140/90:
And still others.
Those who are familiar with the controversy will recognize that there is more nuance than what I am presenting. Presenting the full picture about these nuances would require a post in itself.
But the point is clear:
There is some uncertainty within the international scientific community about what precisely the optimal clinical blood pressure targets should be.
But why should it matter?
Is it possible for blood pressure to be too low?
Why are cutoffs necessary at all?
Why not simply bring blood pressure as low as possible?
One can imagine all sorts of reasons why this might be a bad idea.
But before proceeding, let us clarify a fundamental principle: for cardiovascular disease risk, lower is always better.
These two facts: (1) too low might cause problems and (2) lower is always better for cardiovascular disease risk…
… Are what makes all of this so complicated.
Let us run through through two sets of data: the observational and the genetic data.
This exercise will allow us to understand what the available data indicate, but it will also provide a lesson in how to think about observational data, in general.
Pay close attention.
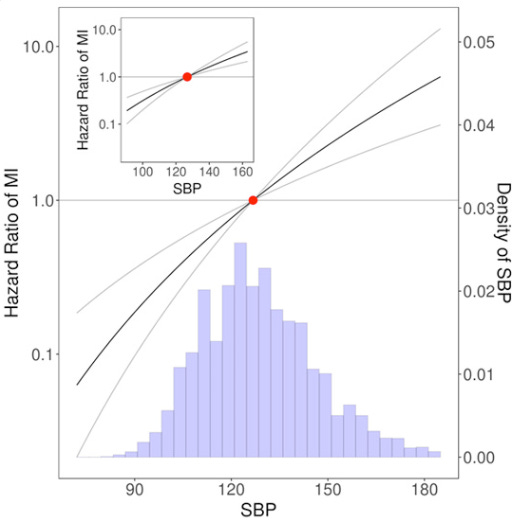
Observational data on systolic blood pressure (SBP)—the first number in a blood pressure figure, i.e. the “120” part of “120/80”—show a very linear relationship between SBP and myocardial infarction (heart attack; one of the major forms of heart disease) risk.
That is, the lower the systolic blood pressure, the lower the risk of heart attack.
The lower, the better.
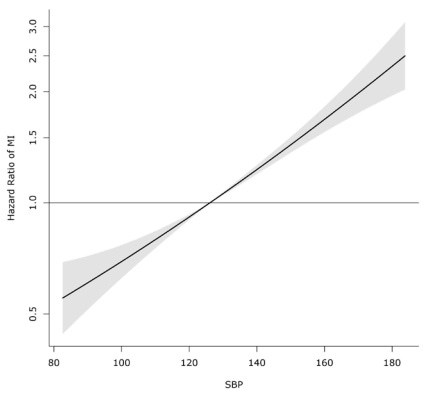
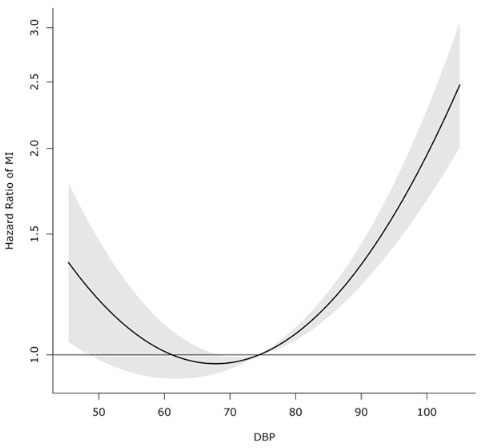
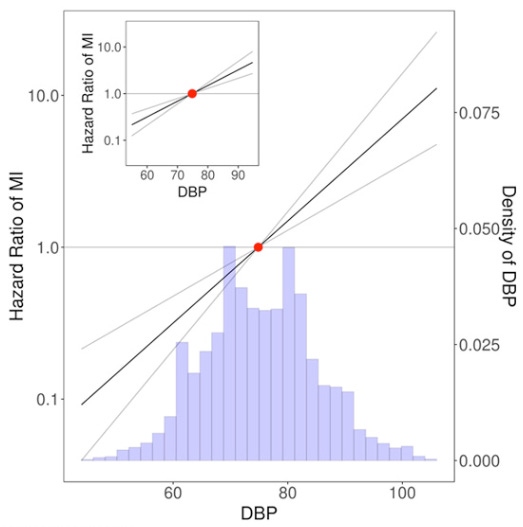
Yet observational data show that this relationship is much more complicated for diastolic blood pressure (DBP)—the second part of the blood pressure figure, i.e. the “80” part of the “120”:
This figure suggest that above about 80 mmHg DBP, the risk of heart disease rises, and below about 60 mmHg DBP, the risk of cardiovascular disease also rises.
So while low SBP is fine, it seems that having too low DBP might actually be harmful.
The sweet spot for DBP appears to be between 60 and 80 mmHg.
Researchers have long suggested that this might be because when the diastolic blood pressure is too low, the heart cannot be adequately perfused and oxygenated. So you don’t want DBP dropping too low, or the heart cannot function properly: hence, heart disease.
But there is another explanation.
It might be that people who develop really low diastolic blood pressure—due to damage of the blood vessels and a loss of elasticity—simply have a higher chance of having heart attacks, simply because low DBP may in many people be a sign of heart disease in the first place.
In other words, it might be that it is not low DBP that causes a higher risk of heart attack; rather, heart disease—which predisposes to heart attack—may be what causes a really low DBP.
But which explanation is true cannot be determined by just looking at observational data.
Observational data can only tell us about correlations. It cannot tell us about causation. That is why we have multiple different potential explanations to explain the data.
What we need is a different kind of study: a kind of study that can determine causation.
We can do that by using a study design called Mendelian randomization. Mendelian randomization allows investigators to look at blood pressure genetics, rather than look at actual blood pressure.
Here’s how it works:
What happens when you look at all of the people genetics for really high DBP? What is their risk for heart attack? What about looking at all of the people with genetics for really low DBP? What is their risk? And what about people with genetics for intermediate DBP? What is their risk?
What happens if we compare the heart disease risk for all of them?
The idea is that environmental factors are excluded—since environmental factors will be evenly distributed between people with low, intermediate, and high genetic DBP. And since environmental factors are evenly distributed, the only factor that we are looking at is DBP caused by genetics—not caused by disease—and its impact on heart disease.
Using Mendelian Randomization, investigators found the same thing with systolic blood pressure as they did with the observational data.
So that confirms it: for heart attack risk, the lower the SBP, the better.
But for DBP, they found something very different than the observational data indicated: for DBP, the lower the better.
There was no association between low DBP and elevated risk.
In other words, Mendelian randomization demonstrates that the observational data was founded. The Mendelian Randomization studies, in short, showed that lower DBP and SBP is always better for heart attack risk.
The same is true of coronary disease, ischemic stroke, heart failure, fatal coronary disease, and all-cause mortality.
Lower is better, when the proper interpretation of the literature is made.
The evolutionary basis for optimal blood pressure in humans
Or is it?
In the above example, we were looking only at the risk of heart disease and death.
But is it possible that low blood pressure, despite reducing the risk of heart attack, might have other downsides?
Obviously.
But first, to frame this question, we must understand why human blood pressure is at the level that it is: the evolutionary reason.
In other words, why does normal blood pressure in humans increase risk of heart disease? Shouldn’t normal blood pressure be perfectly protective against heart disease?
But it isn’t.
Why not?
An evolutionary discussion will provide us with a comprehensive understanding of the complicated tradeoffs in blood pressure management. This will set the stage for a discussion about why the guidelines disagree, and finally, using the clinical trial evidence, how to think dynamically about blood pressure optimization in a way that goes beyond simplistic cutoffs—in way that minimizes disease risk while optimizing function and wellbeing.
A fascinating 2015 paper by a group of physicians in Germany lays out the evolutionary biology of blood pressure well.
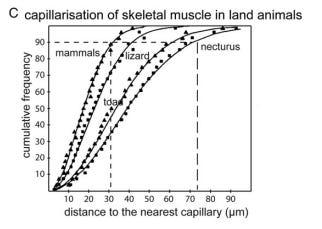
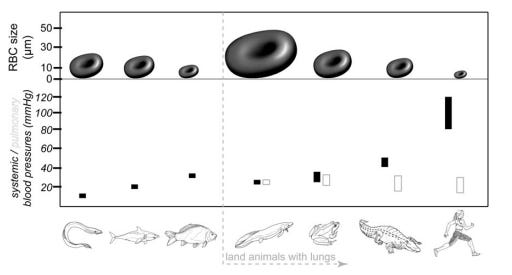
It begins by pointing out that during the evolution of land animals, the diameter of the erythrocyte (red blood cell) shrank 10-fold, from 50 μm to 5 μm.
This was because capillary diameter shrank over the course of evolutionary history. The shrinking of capillaries facilitates more rapid diffusion of oxygen, since oxygen can then diffuse over shorter distances. Furthermore, as capillaries shrank, a denser network capillaries became possible.
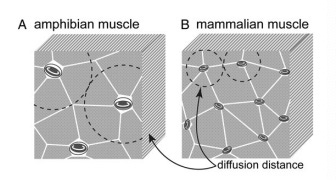
Smaller capillaries capable of more efficient oxygen diffusion, plus a denser network of such capillaries, together enabled a higher rate of oxygen consumption by the organs.
This enabled the development of more metabolically active organisms, with more energetically demanding organs, such as muscles and the brain.
Consider the locomotive differences between a frog and a bird: the former is physically active only for brief periods of time, while the latter is capable of intense, sustained activity in the form of flight.
But similarly, consider the cognitive differences between frogs and birds, too: the former has a very rudimentary nervous system, while some birds, such as corvids (such as crows and ravens), have a brain-to-body ratio similar to non-human primates; scientists are only now discovering that corvids possess an intellectual capacity on the order of monkeys or possibly even gorillas.
Below we can see corvid intelligence in action.
Neither bird locomotion nor corvid intelligence—nor locomotion or intelligence in primates—would have been possible without the “miniaturization” in capillaries that took place over the course of evolution.
What does this mean for blood pressure?
The increase in capillary density also means a higher resistance in the capillary bed. According to the Hagen-Poiseuille equation, a reduction in vessel radius increases vascular resistance to the 4th power (r⁴). A much higher blood pressure is therefore required to perfuse these denser capillary beds.
Consistent with this, consider therefore the typical systolic blood pressures of the following animals:
Fish: 25-50 mmHg
Reptiles: 40-60 mmHg
Mammals: 120 mmHg
Birds: 150-200 mmHg
Two related considerations that the authors consider are as follows.
First, despite the relatively low systolic blood pressure of water animals, gravity poses less of a problem for the adequate blood perfusion of water animals than it does for terrestrial animals. This is because the hydrostatic pressure of the water pushes against the bodies of water animals from all directions, overwhelming the effects of gravity on the vasculature: in water animals, blood simply isn’t pushed down by gravity, but an additional effect is at play: to a degree, blood is pushed back up by the pressure of water.
For land animals, on the other hand, orthostatic hypotension poses a larger problem: gravity causes blood to “pool” at the bottom of the organism, with no countervailing forces upwards. Thus the heart must pump against this gravitational force, with no “help” from the surrounding water.
Therefore, land animals require a larger blood pressure to oppose the impact of gravity on blood flow; correspondingly, mammals tend to have a much higher blood pressure.
Early land animals that are the immediate descendants of water animals—like amphibians and reptiles—have blood pressures somewhere in between water animals and mammals. With a horizontal body plan, reptiles and amphibians have systolic blood pressures in the 40-60 mmHg range: higher than fish (25-50 mmHg) but not as high as evolutionarily later animals like mammals (120 mmHg).
On the opposite extreme of fish, consider the case of humans: not just land animals and not just mammals, but the only bipedal mammals with an upright posture. In humans, blood must habitually fight especially hard against gravity to be pumped upward in a vertical column (the human neck) and perfuse the brain. Hence, humans have a relatively high systolic blood pressure in the range of ~120 mmHg.
The only other bipedal species are birds and dinosaurs, with systolic blood pressures of 140 mmHg and a (posited) 140 mmHg, respectively.
Harvard cardiologist Haider Wairraich in a 2019 New York Times opinion piece speculates about the role of the uniquely aggressive immune system in making humans perhaps specially susceptible to cardiovascular disease. As his speculation goes, an enterprising species that has explored and historically inhabited virtually every corner of the globe (sans Antarctica), humans have evolved an especially aggressive immune system to deal with a veritable kaleidoscope of pathogens that constantly threatened to put an end to the restless adventures of Homo sapiens. In a variant of the hygiene hypothesis—cardiovascular disease style—Dr. Wairraich explains that the Tsimane, who bear a heavy parasite burden, have no cardiovascular disease.
While the role of inflammation in atherosclerotic disease was definitively demonstrated by the CANTOS trial, Wairraich’s hypothesis has many problems—not least of which is that pathogen burden is generally positively correlated with atherogenesis.
A simpler and better supported explanation is at hand.
Human are not the only species that experiences a high incidence of atherosclerosis; incidence of atherosclerosis in a species seems to closely track mean blood pressure in each species.
As the researchers from Germany explain:
In the 1960s, the heart, aorta and central arteries were analysed in 816 animals which had died from natural causes in the London Zoological Garden. Among those animals, there were 485 birds, 233 mammals and 98 reptiles. In 1% of the reptiles, 3% of the mammals and 18% of the birds analysed, arteriosclerosis was present. ... A sub-analysis revealed that the Falconiformes (eagles, hawks etc.), the top performers of all birds with a resting blood pressure of 200 mmHg, were the front runners in developing arteriosclerosis (i.e. ∼54% of the analysed specimens). No arteriosclerosis was seen in penguins (spenisciformes), which have a resting blood pressure of only 92 mmHg. Obviously, a high arterial pressure is needed to meet the metabolic demands of flying. The downside of this ability is the natural affinity to develop arteriosclerosis. In captivity, parrots with a high blood pressure live with a generous supply of food and the best medical care. In these animals, arteriosclerosis reaches an incidence of up to 70–90% and is, together with heart failure, a major cause of death.
Consistent with this, histological studies of the cardiovascular systems of turkeys—whose systolic blood pressure is around 250 mmHg—show universal atherosclerosis by just two years of age, with a higher burden of plaques in turkeys with higher blood pressure than in those with lower blood pressure.
Fascinatingly, giraffes, owing to their long necks—a very long column to pump blood through against the force of gravity—have an even more significant gravitational burden than humans do to adequately perfuse their brains. Correspondingly, their central systolic blood pressures reach as high as 220 mmHg. Researchers have recently documented a number of genetic mutations in genes related to fibrosis that prevent giraffe hearts from undergoing the sorts of deleterious structural changes that would occur at such high pressures in many animals, including humans, with such high blood pressures. They also have thick connective tissue throughout their legs to prevent the pooling of blood—and edema—that would occur in other mammals in such long legs.
It seems that humans’ “special” propensity toward cardiovascular disease is shared by other animals with a similarly high average species blood pressure. Some species have evolved to endure these pressures, but most with such pressures have bear significant cardiovascular costs, including humans.
This propensity is probably exacerbated by the long lifespans of humans—since cardiovascular disease simply has more time to develop in humans than in most other species.
In short, the human body requires higher blood pressure for proper functioning—despite the fact that lower blood pressure improves longevity.
There is, in a word, a tradeoff between optimal functioning and health, and this tradeoff exists only in some species and not others: only some species require a blood pressure for appropriate functioning that compromises long-term cardiovascular health.
Addressing a common but mistaken view of human health and evolution
Here, it is not unimportant to address and refute an alternative point of view. There is a widespread but false assumption among those interested in nutrition—and as we shall see, even among physicians and scientists and guidelines committees, indeed, among most human beings—that if only a given species could return to the ideal conditions of its evolutionary development (what evolutionary biologists call the Environment of Evolutionary Adaptedness), all disease would be eliminated. This belief is incorrect because it assumes that any given species is somehow evolutionarily complete or perfect; that any given species would function perfectly if only provided the right conditions—those to which it had evolved.
But this is not how evolution works: evolution is not a perfect designer but a tinkerer. To take an analogy from computer science, evolution is a programmer who writes a patchwork of programming code incrementally and often very messily building on previous code. Evolution is not, say, a “10X engineer”, i.e. someone who writes code almost as an architect who elegantly designs beautiful buildings. That this view is correct can easily be seen when one takes into account the wide amount of genetic variation that exists even within homogeneous populations: no environment could perfectly suit all individuals of a species if each of those individuals varies significantly genetically.
From an evolutionary point of view, it may well be that the vascular system, originally evolved in the context of low blood pressures, is imperfectly suited for higher blood pressures that are required for mammalian and human life, no matter how many millions of years of mammalian or human evolution take place. The vascular system may simply be an imperfect platform on which mammalian body plans are built.
Several other interpretations are also possible.
One is that evolution would be capable of compensating for the deleterious effects of blood pressure, if evolutionary pressures were substantial. However, because cardiovascular disease kills humans at a much older age than the age at which they reproduce, there may simply inadequate selection pressures on humans to produce adaptations in the cardiovascular system to make these higher blood pressures not deleterious.
Another interpretation is that evolution has provided adaptations that reduce blood pressure—as evidenced by the substantial proportion of the population that is hypotensive—but that countervailing adaptations maintain higher blood pressures in the population. This may be why there is such a wide variation in blood pressure genetics.
Yet another interpretation is that other aspects of lifestyle cause cardiovascular disease—and that higher blood pressures simply make these other aspects more damaging—and that under “ideal” circumstances, there would be little atherosclerosis in human populations despite higher blood pressures.
These interpretations are theoretical. But the practical matter remains: there is a tradeoff between blood pressure lowering and the appropriate functioning of the human organism. The same is true of excessively high blood pressures, which can also disrupt appropriate functioning even in the short term, especially cognitive and athletic performance.
What’s next
The following planned parts of this article series will discuss more about what happens to humans when blood pressure becomes too low—clinical hypotension. Then it will pivot to interpreting the randomized controlled trial literature in light of the preceding discussion—both the benefits and harms shown in this literature. This literature will precisely quantify the above discussion, making it concrete in terms of blood pressure targets. Then the nutritional physiology of blood pressure modification will be discussed, as well as the tradeoffs inherent in the various approaches available. Next, specific intake targets will be discussed; tools will be provided to extract from these targets a reader’s own personalized plan for rapid blood pressure modification using diet. Potential risks will be addressed. These intake targets will be placed in the context of the current guidelines of different countries, especially nutritional recommendations like the DASH diet, as well as the strengths and weaknesses of the current evidence base—clearly articulating where there is scientific uncertainty. In addition to addressing hypertension, there will be a discussion about how to improve hypotension, and what might be the risks and benefits of doing so. Finally, recipes that will achieve different goals palatably will be provided.
If you have found the above discussion useful or interesting, please consider becoming a paid subscriber; a large investment of time is required to write and research these articles.
Your feedback in the comments section is also much appreciated. If you are inclined to leave a comment, please do.



Thank you Kevin, this is one of the most helpful and insightful things I’ve read on the topic of blood pressure. Looking forward to the rest of it!
Kevin, this lecture (link below) explained so much to me about hemodynamics and its role on blood pressure. I enjoyed your first posting on BP and hope that you touch on blood viscosity in future postings. https://youtu.be/dUlFzVnr-aU?si=X6HQoRDArFQVW1qc